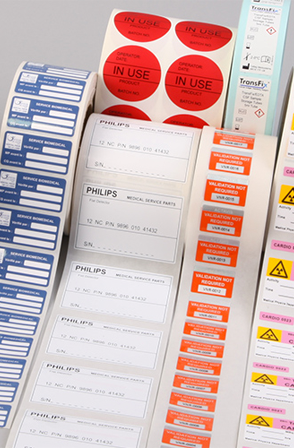
Sensitive information in aggressive environments
Throughout the lifespan of the product, identification and traceability data is subject to various forms of attack able to affect the legibility of the information:Complying with labelling regulations
In the USA for example, the UDI (Unique Device Identification) system requires a unique traceability code, a barcode and a representation of the product on the label for every medical device.
Thermal Transfer enables unitary printing of labels with all types of barcodes, logos, product drawings and other small characters: a guaranteed flexibility for medical device manufacturers.
Medical device manufacturers wishing to sell their products in the USA must submit a Drug Master File to the FDA to validate the safety of all of the products components, including printing inks. ARMOR supports users in this area by making ink composition information available to the FDA.